Chapter 4 Principles of Earth System Science
Climate change is one part of a larger branch of science, called Earth System Science, which views the Earth as a system of parts that are constantly changing and interacting with one another. Different scientists have different ways of describing the Earth system, but one common depiction breaks the Earth down into the atmosphere, consisting of the air, clouds, etc.; the hydrosphere (liquid water in the oceans, seas, lakes, and rivers of the Earth, as well as underground water); the lithosphere, or solid earth, that comprises rocks, dirt, etc.; the cryosphere, accounting for the frozen water (ice and snow); and the biosphere, which contains all living organisms.
4.1 Change in the Earth System
When we study climate change, we mostly concentrate on the atmosphere and the hydrosphere (especially in this course, which emphasizes the connections between climate change, sea-level rise, and floods), but each of the spheres of the Earth system influences all of the others. The chemistry of the atmosphere is strongly influenced by the hydrosphere, the biosphere, the lithosphere, and the cryosphere. Water vapor evaporates from surface water into the atmosphere and falls back down as rain (liquid water, which enters the hydrosphere) and snow (frozen water, which enters the cryosphere). Plants use photosynthesis to remove carbon dioxide (CO2) from the atmosphere and metabolic processes in animals, plants, fungi, and other living things return CO2 and other chemicals to the atmosphere. Volcanic activity in the lithosphere releases CO2 and aerosol particles such as dust and ash into the atmosphere, and chemical reactions associated with the weathering of rocks and minerals removes CO2 from the atmosphere and converts it into chalk, limestone, and other solid forms in the lithosphere.
4.1.1 Balance and Stability in Changing Systems
Before diving into the details of climate change, let us consider more generally what change means in the dynamic and constantly changing Earth system. There are different kinds of change, and even in a constantly changing and evolving system, some things can remain steady and relatively constant. Here, the concepts of equilibrium or steady state are important. Our bodies are constantly changing as we eat, drink, breathe, and excrete. You are constantly breathing in to bring oxygen to your blood and breathing out to remove carbon dioxide. Yet despite the constant change of adding oxygen and removing CO2, the amount of oxygen and CO2 in your blood does not change very much. While many people worry about weight change, it is possible to eat a couple of thousand calories every day without gaining weight. And in the Earth system, it is always raining or snowing somewhere in the world, but the amount of water vapor in the atmosphere does not change very much.
In all of these examples, we see a system remaining in a steady state despite constant change. The key is that there is balance in the change. Your body’s metabolism converts oxygen to carbon dioxide and your nervous system synchronizes your breathing to your metabolism so the oxygen you breathe in and the CO2 you breathe out balance the metabolic conversion of oxygen to CO2. This is why you breathe harder when your metabolism is operating more intensely, such as when you exercise. Your metabolism consumes energy and if you only eat as much as your metabolism consumes, you will not gain weight. Water evaporates into the atmosphere from oceans and other bodies of surface water to balance the water that falls out of the atmosphere as rain or snow.
When we study climate change, we will consider two very important forms of balance that contribute to a steady state of the climate system:
4.1.2 Heat and Temperature [#heat-temperature}]
We use the term heat colloquially without thinking much about what it means, but to scientists, heat has a very specific meaning. It is the spontaneous transfer of energy from something hotter to something colder. There are many kinds of energy transfer, but heat is a transfer that happens spontaneously and that occurs because of the temperature difference between two things or two places.
When you are making iced tea and drop ice cubes into hot tea, heat flows from the hot tea into the cold ice cubes, making the ice cubes warmer and the tea colder. You only see heat flow in this direction, from hot to cold. You never see a glass of lukewarm tea spontaneously turn into cold ice cubes and hot tea.3
Temperature is determined by the amount of thermal energy a thing contains, and its temperature changes when its thermal energy changes. If thermal energy is flowing both into and out of an object, its temperature will rise if there is more thermal energy flowing into it and it will fall if there is more thermal energy flowing out of it.
In the next chapter, we will investigate why the Earth has the temperature it does, and how the greenhouse effect modifies the climate. We will see that the temperature of the Earth is controlled by a balance of heat flowing from the sun to the Earth and heat flowing from the Earth to outer space. If more heat flows from the sun to the Earth than from the Earth to space, the Earth will get warmer (its temperature will rise), and if more heat flows from the Earth to space than from the sun to Earth, the Earth will become cooler (its temperature will fall).
4.1.3 The Biogeochemical Carbon Cycle
The amount of carbon dioxide in the atmosphere is also controlled by a balance of chemical processes that remove CO2 and processes that add it. Photosynthesis, the chemical weathering of rocks and minerals, and dissolving into surface waters are three processes that remove CO2. Respiration (by animals and plants), decomposition of dead organic matter by bacteria and fungi, fires, volcanic activity, and outgassing of dissolved CO2 from surface water to the atmosphere are among the processes that add CO2 to the atmosphere.
For several thousand years before the industrial revolution, the amount of CO2 in the atmosphere was very steady at about 2.2 trillion metric tons. Every year, about 770 billion metric tons of CO2 would be removed by biological and chemical processes (photosynthesis, weathering, etc.) and about 770 billion metric tons would be added by complementary processes (respiration, fire, volcanism, etc.)
For thousands of years, the amount of carbon dioxide released into the atmosphere by biological and chemical processes balanced the amount removed by other biological and chemical processes and the amount of CO2 remained very steady. It might change a bit from year to year, but the changes were very small and
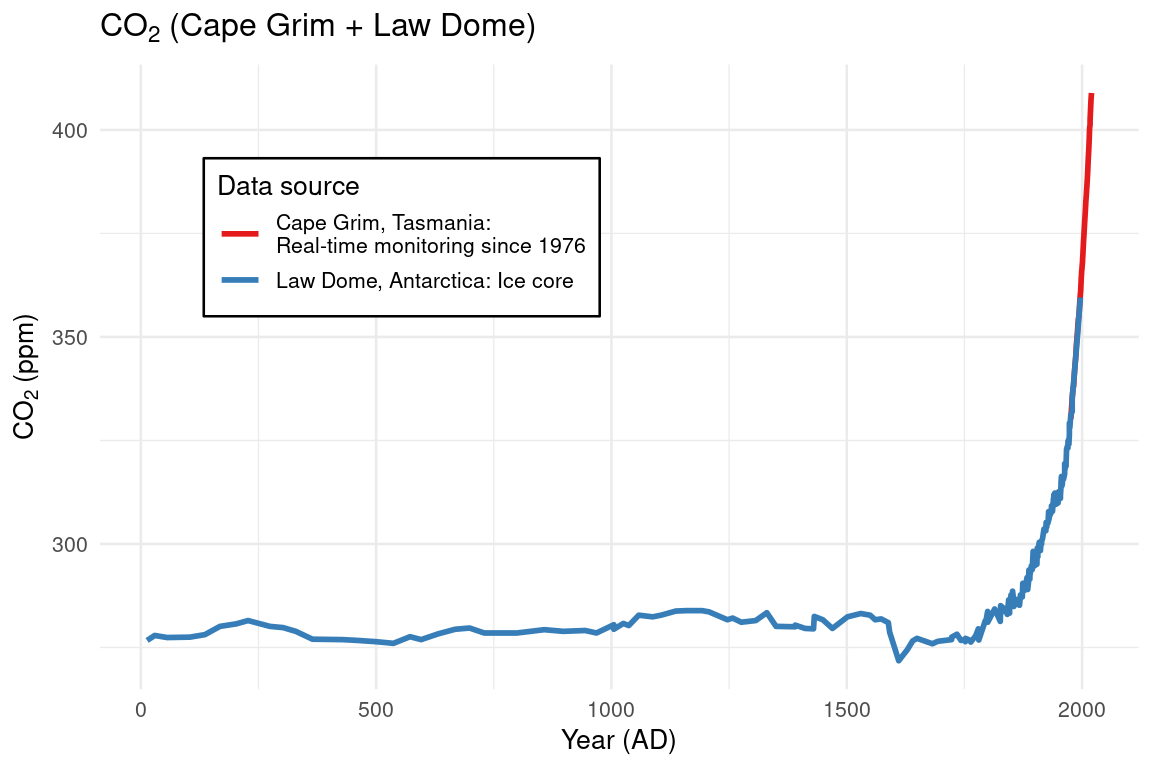
Figure 4.1: Atmospheric levels of carbon dioxide over the past 2000 years. The blue line shows records of CO2 recorded in bubbles in the ice of the Law Dome glacier in Antarctica. (Etheridge et al. 2010; MacFarling Meure et al. 2006) The red line shows real-time measurements of CO2 recorded at the environmental monitoring state at Cape Grim, Tasmania. (Cape Grim Baseline Air Pollution Station 2020) CO2 changed very little until the industrial revolution in the mid-18th century, but subsequently rose by 46%.
Figure 4.1 shows carbon dioxide concentrations in the atmosphere remaining relatively constant for more than 1700 years, but rising rapidly once the industrial revolution began in the mid-18th century. This is because all the natural processes that add and remove CO2 are in balance, but there is nothing to balance the release of CO2 from burning fossil fuels.
4.2 Summary
The Earth system is constantly changing, but attributes of the system, such as temperature and the atmospheric concentration of carbon dioxide may remain relatively steady over long periods of time so long as there is a balance between quantities entering and leaving their part of the Earth system. Temperature remains steady so long as the heat coming in equals the heat going out. CO2 concentrations remain steady so long as the amount of carbon entering the atmosphere balance the amount leaving the atmosphere. For thousands of years, such a balance existed, but when people began burning billions of tons of fossil fuels per year, this source of CO2 was not balanced by a complementary process to remove it, so CO2 built up in the atmosphere.
References
Cape Grim Baseline Air Pollution Station. (2020). Monthly mean baseline carbon dioxide concentration, Cape Grim, Tasmania: Commonwealth Scientific and Industrial Research Organisation (CSIRO). Retrieved from http://capegrim.csiro.au/GreenhouseGas/data/CapeGrim_CO2_data_download.csv
Etheridge, D., MacFarling Meure, C., Trudinger, C., … Elkins, J. (2010). Law Dome ice core 2000-year CO2, CH4, and N2O data (Data No. 2010-070), Boulder, CO: IGBP PAGES/World Data Center for Paleoclimatology. Retrieved from https://www.ncdc.noaa.gov/paleo-search/study/9959
MacFarling Meure, C., Etheridge, D., Trudinger, C., … Elkins, J. (2006). Law Dome CO2, CH4 and N2O ice core records extended to 2000 years BP. Geophysical Research Letters, 33(14). doi:10.1029/2006GL026152
Refrigerators and air conditioners can move heat around to make the inside colder and the outside hotter, but they do so using mechanical work, not the spontaneous flow of heat.↩︎